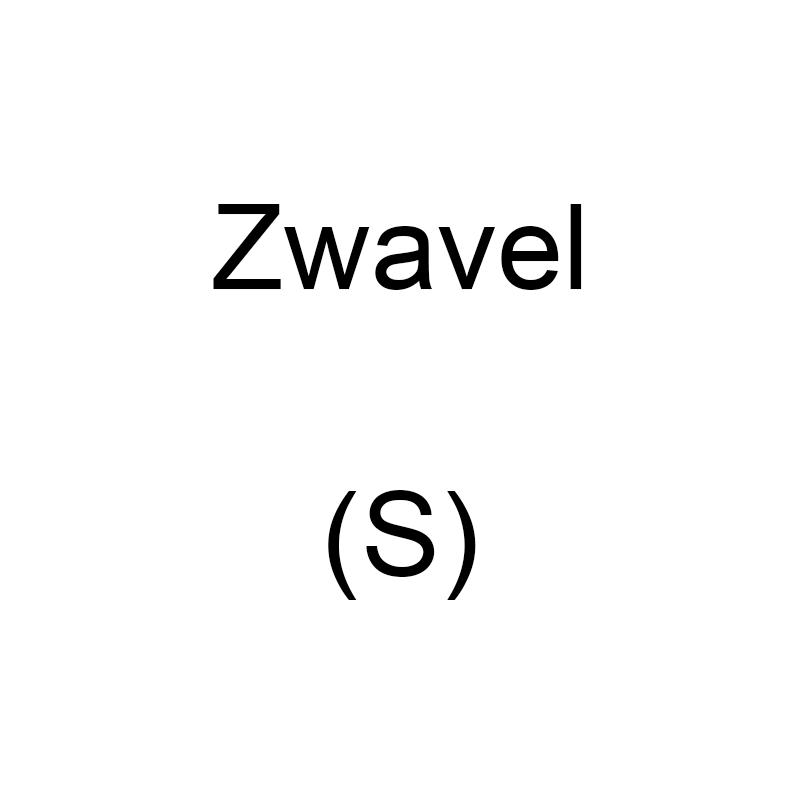
Sulfur
Sulfur is a yellow element with various applications in laboratory settings, from chemical synthesis to catalytic processes.
Vanaf €50 in NL

Stuur ons een bericht

Vraag een offerte aan

Ma – Vr: 9:00 – 17:00
Description
What is Sulfur?
Sulfur is a chemical element with the symbol S and atomic number 16. It is an essential element found in nature in mineral form and in living organisms. With a characteristic bright yellow color, sulfur is known for its unique properties and wide application possibilities in science and industry.
Properties of Sulfur
Some characteristic properties of sulfur are:
- Well soluble in carbon disulfide.
- Burns with a blue flame and releases sulfur dioxide.
- Is not soluble in water.
Applications of Sulfur in Laboratories
Sulfur has several applications in the laboratory, including:
- Production of sulfur salts such as sulfates.
- As a catalyst in various chemical reactions.
- In the manufacture of organosulfur compounds.
FREQUENTLY ASKED QUESTIONS
- What is the best way to store Sulfur? Sulfur should be stored in a tightly sealed container in a cool, dry place, protected from direct sunlight and heat.
- Where can I find more information about Sulphur? For detailed information on sulfur, visit Pubchem.
Where can I buy high quality sulfur for laboratory use?
Are you looking for pure sulphur that is perfectly suited for laboratory research and experiments? We offer high-quality sulphur, ideal for a range of scientific applications, from sulphur salt production to organic synthesis.
Related products
-
Molybdenum
Top quality molybdenum powder, ideal for various laboratory experiments. Experience the chemical versatility and stability of our molybdenum for optimal results.Rated 0 out of 5 -
Zinc
High quality zinc pellets, ideal for chemical experiments and industrial applications. This versatile metal offers excellent catalytic capabilities and corrosion-resistant properties. Perfect for various laboratory and manufacturing processes.€ 8,95 – € 34,95 incl. BTWRated 4.00 out of 5Select options This product has multiple variants. The options may be chosen on the product page




Reviews
There are no reviews yet