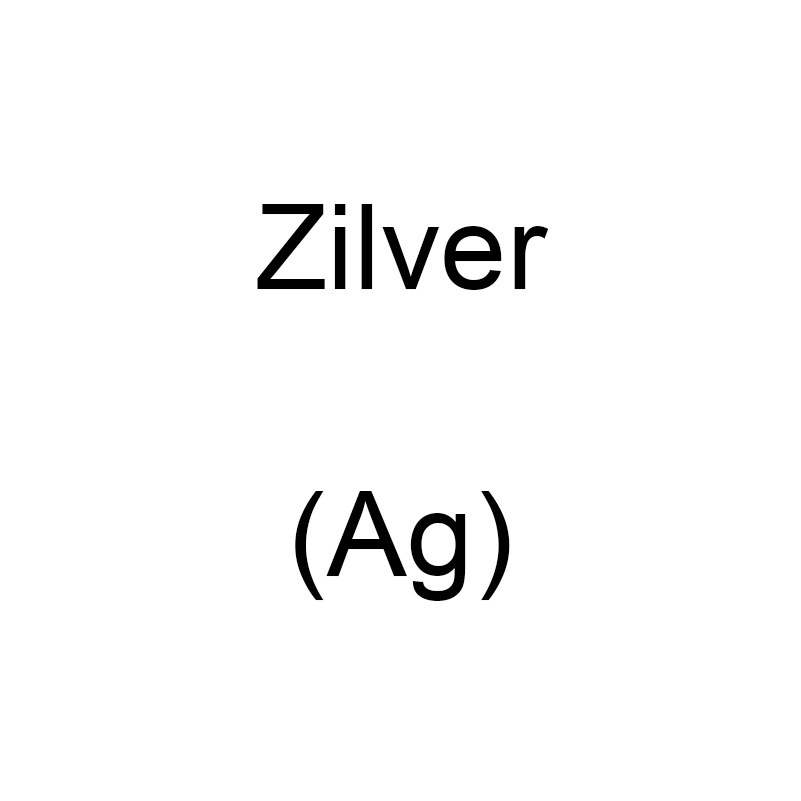
Silver
Silver, a white shiny transition metal, plays an essential role in laboratory applications thanks to its excellent conductive and catalytic properties.
Vanaf €50 in NL

Stuur ons een bericht

Vraag een offerte aan

Ma – Vr: 9:00 – 17:00
Description
What is Silver?
Silver is a chemical element with the symbol Ag and atomic number 47. It is a soft, white, shiny transition metal that has been appreciated since ancient times for its ornamental value and its many applications. The metal is known for its high-quality conductive properties.
Properties of Silver
Some characteristic properties of silver are:
- Excellent electrical conductivity.
- Antimicrobial properties.
- High melting point and reflective capacity.
Applications of Silver in Laboratories
Silver has several applications in the laboratory, including:
- As a catalyst in chemical reactions.
- In electrochemical studies due to its conductive properties.
- For the production of certain silver salts and compounds.
FREQUENTLY ASKED QUESTIONS
- What is the best way to store Silver? Silver should be stored in a cool, dry place and protected from exposure to sulfur-containing materials to prevent discoloration (blackening).
- Where can I find more information about Silver? For detailed information about silver, visit Pubchem.
Where can I buy high quality Silver for laboratory use?
Looking for silver suitable for laboratory studies and experiments? With us you will find high quality silver that is ideal for various scientific applications, from catalytic studies to the production of specific silver compounds.
Related products
-
Zinc
High quality zinc pellets, ideal for chemical experiments and industrial applications. This versatile metal offers excellent catalytic capabilities and corrosion-resistant properties. Perfect for various laboratory and manufacturing processes.€ 8,95 – € 34,95Price range: € 8,95 through € 34,95 incl. BTWRated 4.00 out of 5Select options This product has multiple variants. The options may be chosen on the product page -
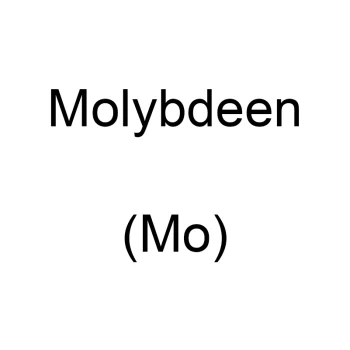
Molybdenum
Top quality molybdenum powder, ideal for various laboratory experiments. Experience the chemical versatility and stability of our molybdenum for optimal results.Rated 0 out of 5



Reviews
There are no reviews yet