What is Aluminum?
Aluminium is a light, durable and silver-white metal. Because of its reactivity and conductive properties, aluminium is often used in chemical reactions and experiments.
Shapes of Aluminium
We offer aluminium in the following specific forms for chemical applications:
- Granules: This shape is ideal for certain reactions where a standardized size and shape are important.
- Powder: Powdered aluminum is often used in exothermic reactions and as a reducing agent in certain chemical processes.
Chemical Applications of Aluminum
Due to its unique properties, aluminum is sought after in chemical processes, including:
- As a reducing agent in organic and inorganic synthesis.
- For producing hydrogen gas in reaction with acids.
- As a component in certain pyrotechnic mixtures.
FREQUENTLY ASKED QUESTIONS
- How should I store Aluminium safely? Store in a dry place. Especially the powder form should be kept away from ignition sources.
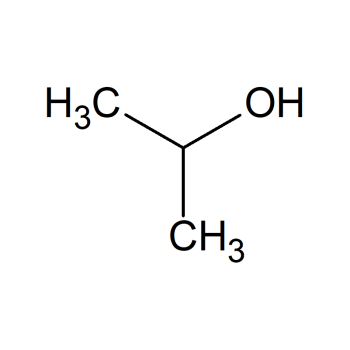
- Can Aluminium react with water? Yes, aluminum can react with water to produce hydrogen gas, although the reaction can be slow depending on the conditions.
- Where can I find more information about Aluminium? More information can be found in the safety data sheets offered, or at: Pubchem.
Where can I buy Aluminium in Granules and Powder form for chemical applications?
Are you looking for aluminium specifically for chemical reactions and experiments? DutchChems offers Aluminium in both granular and powder form suitable for various chemical processes.

















Reviews
There are no reviews yet